
GEM Abstraction Manual
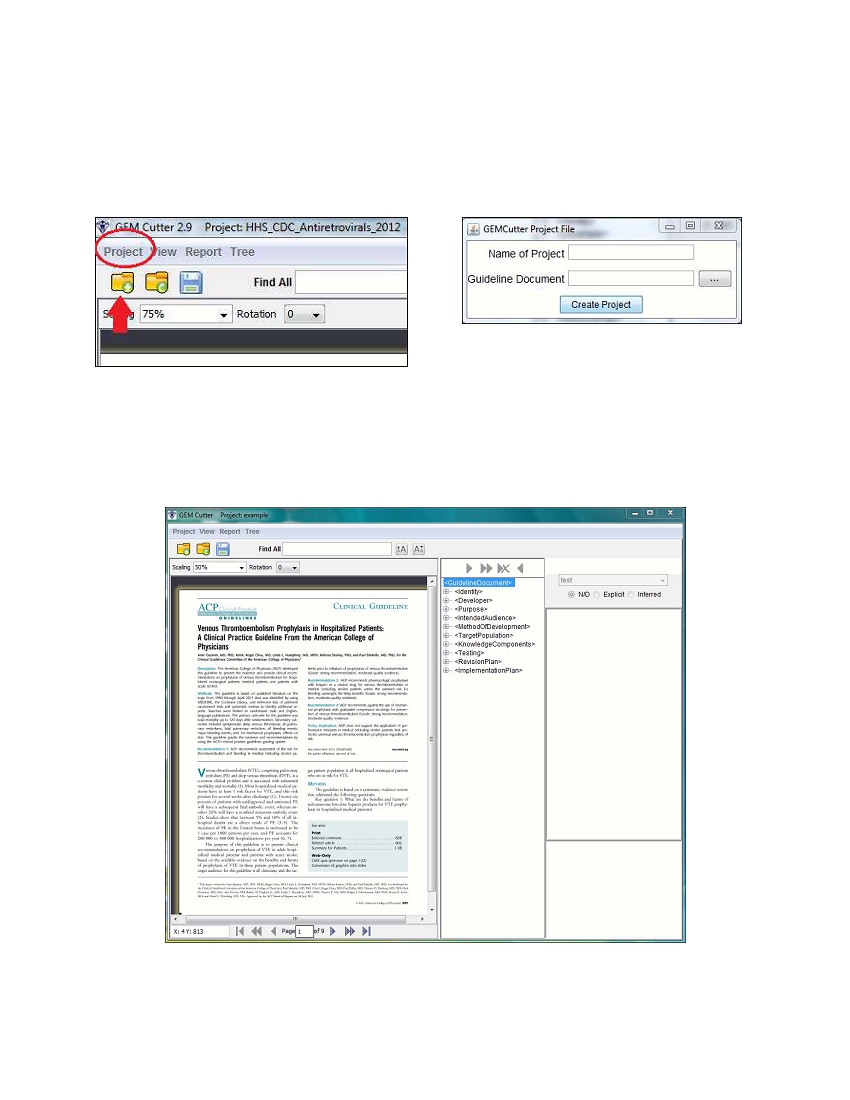
To start using GEM Cutter, go into the folder named “GEM Cutter III” and double-click on the Executable Jar
File “GemCutter.jar”, as shown below.
This will launch the GEM-Cutter application and the main screen below will appear.
Beginning a New Project
To begin cutting a guideline for the first time, click on “Project” at the top left of the menu bar and then
select “New Project” from the drop-down menu. Alternatively, you can click on the “New Project” icon.
(below left). The window shown at below right will appear.
Enter a name for the project which will permit easy identification. The use of spaces is not supported—use
underscore (_) if necessary. Next click on the button with the ellipsis (…), which will allow you to browse
your computer to find the file of the guideline you will be cutting. After you have identified and selected the
full-text file of the guideline, click “Create Project”. The guideline document is loaded into the cutter and
will be visible in the leftmost of the three main panels:

The middle panel displays a tree view of the GEM hierarchy, containing ten “top-level” elements (below
left), which can be collapsed and expanded using the plus and minus icons (below middle). There is also a
“Tree” feature available from the menu bar (below right), which provides two dropdown items: Expand All
and Collapse All, which can be used to display all or none of the elements in the GEM tree (eliminating the
need to individually/manually expand and collapse the items. If the tree is fully collapsed, double-clicking
<GuidelineDocument> will show the ten next level elements.
The GEM hierarchy provides the ability to capture over 100 “elements”, or unique types of information
about the guideline. A listing of the elements and their definitions are listed below. A checkmark (“ ”)
denotes an element currently required to be GEM-cut. Note that elements containing coding information
(e.g. Inclusion Criterion Code, Decision Variable Code) will be populated by indexers rather than GEM
abstractors.
Identity
Record of GEM file creation
Identification details of the current instance of the GEM file
o
GEM Cut Author
Individual responsible for creating this version of the GEM file
o
GEM Cute Date
Date when this version of the GEM file was created
Complete title of the guideline

Bibliographic citation
Number of pages in printed document
Date the guideline was released to the public
Information regarding sources of a guideline and associated
Availability
documentation
Information regarding sources of guideline in electronic format
Information regarding sources of guideline in print format
Person or organization to contact for additional information
about a guideline
Statement of whether the guideline is original or a revised or
Status
updated version of a previously issued document
Refers to other documents (including Technical Reports,
Companion Document
Consumer Guidelines, Quick Reference Guidelines) produced by
the guideline developer relevant to the guideline
A patient-oriented summary of guideline content or a resource
intended to assist patients with guideline application.
A concise document that summarizes guideline
recommendations for clinicians
A document or document component that describes in detail
the method of guideline development
Indicates that the guideline has been adapted from another
Adaptation
guideline
A summary statement that describes a guideline using
Structured Abstract
structured headings
Developer
Organization(s) responsible for developing the guideline
Formal name of committee within developer organization
Committee Name
responsible for developing guideline
Expertise present within the group that authored guideline
Name of member of guideline development committee
A potential source of bias (e.g., financial or intellectual) related
o
Member Conflict
to a panelist or potential panelist that could influence the CPG
development process
Expected function of a committee member, e.g., chair,
o
Member Role
epidemiologist, or implementation specialist
Professional expertise of individual guideline committee
o
Member Expertise
member
Funding
Source of financial support for guideline development
Endorser
Organization that has endorsed the guideline
Comparable Guideline
Another guideline on the same or similar topic
The sponsor’s part in developing, modifying, and reporting the
Role of Sponsor
guideline
Potential situations in which financial or other considerations
Conflict of Interest
may compromise, or have the appearance of compromising, a
developer’s professional judgment
Principles and strategies adopted by developer to address
potential conflicts
Report of potential and real conflicts of interest and how they
are addressed
Purpose
Primary disease/condition, treatment/intervention, health
Main Focus
practice, service, or technology addressed in the guideline main
focus
Reasons for developing recommendations including why the
Rationale
guideline was developed/needed, e.g., evidence of practice
variation or inappropriate practice
General goals that implementation of the guideline is intended
to bring about
Principal alternative preventive, diagnostic, or therapeutic
Available Option
strategies considered
The most important specific outcomes (health, economic, etc)
Health Outcome
considered in the guideline
Situations in which socially relevant factors permit an exception
Exception
to be made in applying the guidelines; including home and
family situation, constraints on health care delivery system
Intended Audience
Intended users of guideline information
The settings in which the guideline is intended for use
Method of Development
Role of patients, advocates, consumer organizations in
guideline development and review
A description of methods used to collect, identify, and retrieve
scientific evidence on each question on which
Description of Evidence Collection
recommendations are based, including details on computer
searches (including dates) and use of personal files and
bibliographies
Publication date of earliest and most recent evidence
considered
Number of source documents identified
Methods used to choose the evidence that informs guideline
development, including inclusion and exclusion criteria for
specific studies
Method of synthesis used to combine the scientific evidence
Description of Evidence Combination
quantitatively or otherwise
Cost Analysis
Describes any formal cost analysis performed

Qualitative description of anticipated benefits and potential
Specification of Harm(s) and Benefit(s)
risks associated with implementation of guideline
Quantification of benefits or risks associated with
Quantification of Harm(s) and Benefit(s)
implementation of guideline
Implicit or explicit process for judging relative desirability of
Role of Value Judgment
health, economic, and process outcomes associated with
alternative practices
Role of patient preferences for possible outcomes of care when
Role of Patient Preference
the appropriateness of a clinical intervention involves a
substantial element of personal choice or values
Important caveat relating to a major recommendation.
Qualifying Statement
Identifies an area of uncertainty
Group judgment techniques used to reach judgment on
Methods to Reach Judgment
recommendations; a description of how the developer made
the transition from evidence to recommendation
Criteria for rating quality of evidence and/or strength of
recommendation
Criteria for rating quality of evidence
Criteria for rating strength of recommendation
Scheme
Target Population
Describes population that the recommendations are intended
to affect; identifies restrictions on guideline use such as within a
managed care organization or geographic region
A criterion whose presence is necessary for the guideline
recommendations to be applicable
An identifier selected from a standard terminology that
o
Inclusion Criterion Code
describes an inclusion criterion
A criterion whose presence excludes the applicability of the
recommendations
An identifier selected from a standard terminology that
o
Exclusion Criterion Code
describes an exclusion criterion
Knowledge Components
A non-executable statement intended by the author to describe
appropriate care. This category includes US Preventive Services
Task Force “I Statements”, i.e., the authors conclude that there
is insufficient evidence to support a recommendation for or
against such an action
Statement of appropriate practice and the conditions under
which it is to be undertaken. The statement is intended to
influence practitioners' behavior and/or patient outcomes. A
number or brief title for a specific recommendation should be
stored in this element.
Additional comments related to the development of the
recommendation

A recommendation applicable under circumstances specified by
an if-then statement. The complete text of the conditional
statement should be stored in this element
The process (including values applied) and the outcome of
o
Benefit Harm Assessment
weighing benefits against risks, harms, and costs that expresses
equilibrium or net benefit or harm.
A condition that must be tested to indicate the appropriateness
o
Decision Variable
of a conditional recommendation. Store only a single variable in
each decision variable element
Identifier selected from a standard terminology that describes a
decision variable
A specified state of a decision variable
Text that provides and amplifies information about a decision
Description
variable
Information about the quality of a decision variable
An indication of the probability of the decision variable being
< Sensitivity
present under specific clinical circumstances
An indication of the probability of the decision variable being
< Specificity
absent under specific clinical circumstances
An indication of the probability of an outcome occurring when a
< Predictive Value
particular value of the decision variable is present
The cost of testing a decision variable
Appropriate activity to be carried out given the specific
o
Action
circumstances defined by values of decision variables. Store
only a single action in each Action element
An improvement in status of some measured outcome that may
occur as a result of following a recommendation
Risk or adverse outcome associated with a specified action
The person(s) or role intended to carry out the recommended
activity
The word or phrase in a recommendation that expresses action,
state, or relationship
The word or phrase that defines the level of obligation of an
active or directive
Word or phrase that completes the sense of a verb and includes
Complement
direct and indirect objects
Identifier selected from a standard terminology that describes
an action or directive
Text that provides and amplifies information about an action
< Intentional
An indication of the reason for deliberate underspecification of
Vagueness
a recommendation’s conditions or actions
Cost of performing a specific action
A specified state of an action
A categorization of activity directed by a conditional

o
Reason
An explanation or justification for a recommendation
An indication of methodologic rigor of the studies that support
o
Evidence Quality
the specified recommendation
Description of the applicability, quantity (including
completeness) and consistency of the aggregate available
evidence. It may include an explanation of the part played by
Description
values, opinion, theory, and clinical experience in deriving the
recommendation
Description and explanation of any differences of opinion
regarding the recommendation, including minority report
An indication of the guideline developers' level of support for a
o
Recommendation Strength
given recommendation
Identifier selected from a standard terminology that describes
Strength Code
the recommendation strength
o
Flexibility
Indication of options in performing imperative
Boolean operators that indicate how directives are to be
o
Logic
combined
o
Cost
Overall cost of performing this recommendation
Indicator of a relationship between this recommendation and
o
Linkage
other knowledge component(s)
o
Reference
Specific citation relevant to this imperative recommendation
Indication of the likelihood that this recommendation will lead
o
Certainty
to specified outcomes
The state that a recommendation is intended to achieve,
o
Goal
maintain, or avoid
Recommendation directed at the entire target population
without limitation. The complete text of the imperative
statement should be stored in this element
The process (including values applied) and the outcome of
o
Benefit Harm Assessment
weighing benefits against risks, harms, and costs that expresses
equilibrium or net benefit or harm.
o
Scope
Implicit eligibility criteria for an imperative statement
Identifier selected from a standard terminology that describes
the scope
An appropriate activity for the eligible population. Store only a
o
Directive
single activity in each Directive element
An improvement in status that may occur as a result of
following a directive
Risk or adverse outcome associated with implementation of a
directive
The person(s) or role intended to carry out the recommended
activity
The word or phrase in a recommendation that expresses action,
state, or relationship

The word or phrase that defines the level of obligation of an
active or directive
Word or phrase that completes the sense of a verb and includes
Complement
direct and indirect objects
Identifier selected from a standard terminology that describes
an action or directive
Text that provides and amplifies information about a directive
< Intentional
An indication of the reason for deliberate underspecification of
Vagueness
a recommendation’s conditions or actions
Cost of performing a specific directive
The specified state of a directive
A categorization of activity directed by an imperative
o
Reason
An explanation or justification for a recommendation
An indication of methodologic rigor of the studies that support
o
Evidence Quality
the specified recommendation
An indication of the guideline developers' level of support for a
o
Recommendation Strength
given recommendation
Identifier selected from a standard terminology that describes
Strength Code
the recommendation strength
o
Flexibility
Indication of options in performing imperative
Boolean operators that indicate how directives are to be
o
Logic
combined
o
Cost
Overall cost of performing this recommendation
Indicator of a relationship between this recommendation and
o
Linkage
other knowledge component(s)
o
Reference
Specific citation relevant to this imperative recommendation
Indication of the likelihood that this recommendation will lead
o
Certainty
to specified outcomes
The state that a recommendation is intended to achieve,
o
Goal
maintain, or avoid
Concise description of terminology relevant to the guideline
A word or phrase defined in the guideline
Precise meaning of words and phrases that may be unfamiliar
o
Term Meaning
to guideline readers; terms are defined as used in this guideline
context
A flowchart representation of the stages and activities in health
Algorithm
management described by the guideline
Specifies clinical actions that are to be performed in the patient-
care process (GLIF)
Directs flow from one guideline step to another based on the
evaluation of a criterion (GLIF)
Directs flow in alternate directions (GLIF)

Synchronization Step represents a convergence of other steps
(GLIF)
Proposal for further scientific investigation to correct identified
Research Agenda
deficiencies in the evidence base for this guideline topic
Information relevant to the guideline’s topic but not related to
Background Information
other Knowledge Components
Testing
Methods of eliciting peer review comments and vetting
External Review
guideline draft
Pilot Testing
Preliminary validation testing
Evaluation of the guideline draft to appraise its validity and
Formal Appraisal
usability (e.g., COGS), quality (e.g., AGREE) and
implementability (e.g., GLIA)
Revision Plan
Expiration
Time (or date) that recommendations cease to be valid
Future time (or date) planned to review continued
Scheduled Review
appropriateness of recommendations
Implementation Plan
Implementation Strategy
Specific plans for implementing the recommendations
A factor that might be expected to impede operationalization of
Anticipated Barrier
the guideline
A factor that might be expected to promote operationalization
Anticipated Enabler
of the guideline
Guideline-derived tool to measure the quality of care they
Performance Measure
provide by defining specific, measurable elements
Starting to GEM-Cut
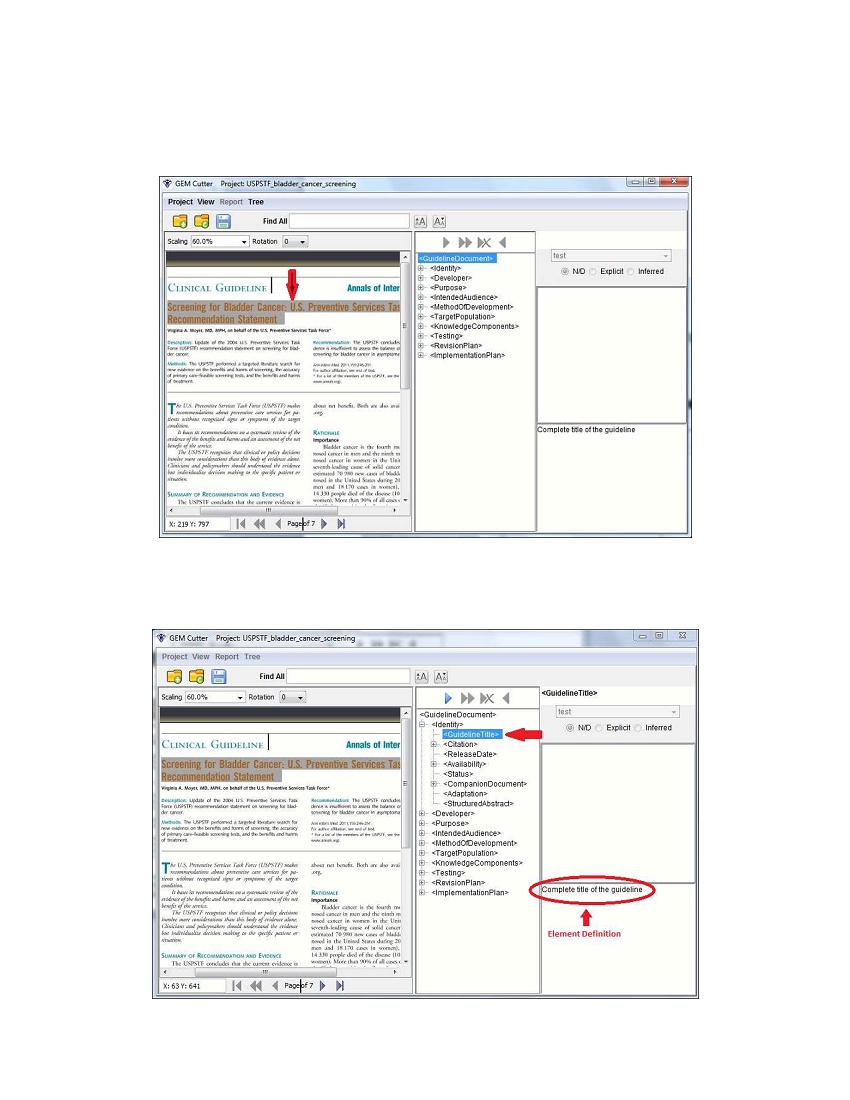
To get started, identify guideline text that you would like to cut, then select it as shown below:
Next, select the element in the tree view that pertains to this text, e.g., <GuidelineTitle> as shown below.
Note that in the bottom right-hand corner a definition of the element selected will be displayed, which can
be used to ensure that you are capturing the appropriate text for that element.
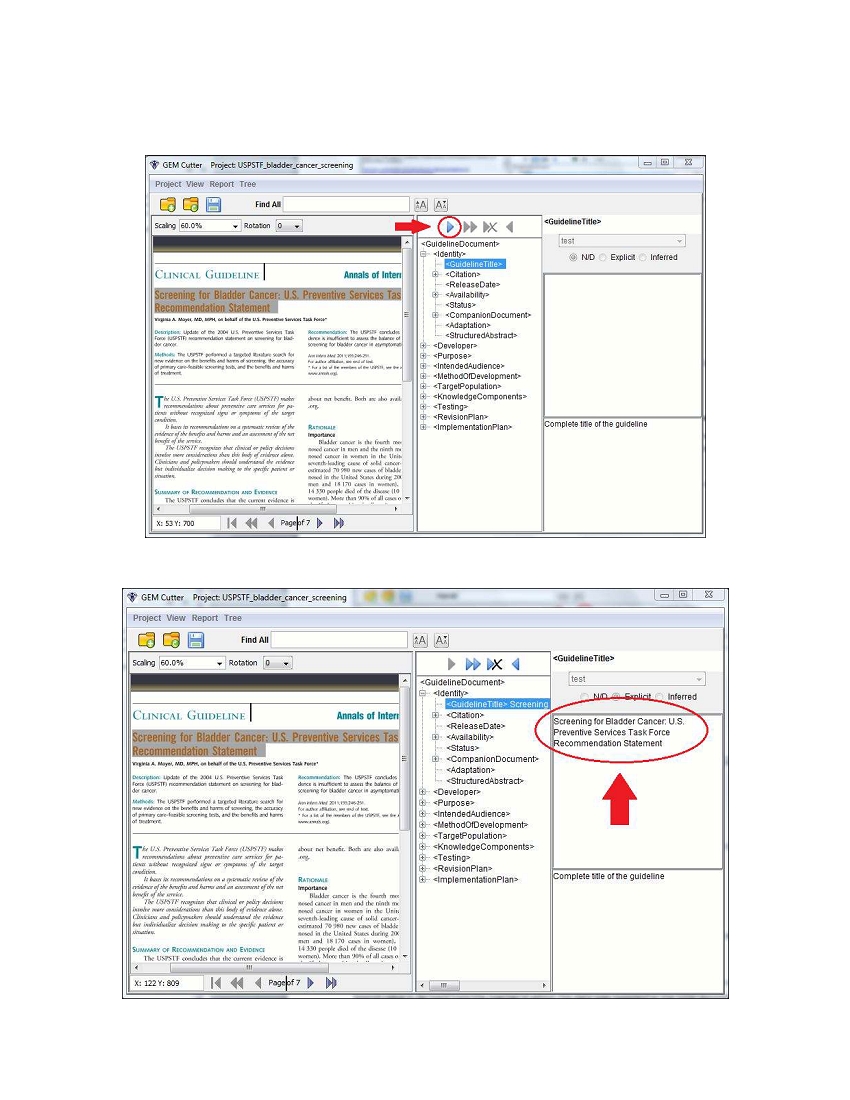
Finally, click on the first arrow button as shown below (“hovering” with your mouse above this button will
read “Move Text”).
The text will be moved into the text box on the right.

You can edit the text in the text box, if you wish to make changes to the original guideline text. If you choose
to edit text, the button above the text box will move to Inferred, indicating in the XML document that this
text is not the original, but has been modified. In addition to the “Move Text” button, the additional below
features are available:
1. Move Text - Inserts the currently selected text into the tree view
2. Append Text - Adds the selected text to the end of the existing element text
3. Overwrite Text - Replaces the existing text in the tree view with the selected text
4. Remove Text – Erases the contents of the element in the tree view
5. Create Subtree – Makes a copy of the currently selected element in the tree view and its child elements
6. Remove Subtree – Deletes the currently selected element in the tree view and its child elements
7. Logic - Displays the Logic Window to assist in writing to the Logic Element. Detailed instructions for
using the Logic feature are provided below in this document.
Populating the Logic Element
The Logic button (#7 in the above diagram), will become “active” if the Conditional or Imperative element
in the tree is selected. By clicking on the button, the Logic Window, shown below, will be displayed.

This window displays an "If" box, which contains a list of the decision variables for recommendations cut as
Conditional (this box is blank for Imperatives). To the left is a vertical row of buttons that will insert the
text on the button where the cursor is located in the text area. The same applies to the lower "Then" box,
but here the Actions are listed for for a Conditional, Directives for an Imperative. To Save the logic, select
the Save button in the lower right.
Saving and Reopening a Project
When you are finished GEM-cutting, to save the file, select Project from the menu bar and then “Save Project”.
Alternatively, you can click on the icon of the disk (below left). A screen like the one at below right should
appear:
This action saves the project file as well as the GEM XML file. You can continue to GEM-cut the guideline, or
exit the program. To use the program with a previously created project file, select Project and the Open
Project menu item. The screen below will appear:
Click on the button with the ellipsis and navigate to your existing project folder. Select the file with .zip
attached to the name of your project and press the Select button. Now select the Open Project button
shown above. You should now be able to work on your previously started project.
Generating Reports
When you are finished GEM-cutting a guideline, the GEM-Cutter tool provides the ability to generate a
variety of different reports, which capture and display the guideline information in different ways. The
third menu bar item is Report. This item contains six menu choices.
1. Extractor-Detailed . By selecting this view, a screen showing the extracted version of the current GEM
document is displayed.

2. Extractor-Rules . In this report the guideline recommendations are stated in full and parsed into
imperative and conditional statements, followed by a restatement as IF…THEN rules (IF decision
variables…THEN actions) or as directives (IF a member of the Target Population…THEN directive).
Blocks are included in the report in which decidability and executability can be commented upon and
vocabulary codes for the parsed components may be entered.

3. Decision Variables . In this report, all of the decision variables are removed from guideline context and
presented in a list. This report offers an enhanced opportunity to judge and identify vagueness,
underspecification, and decidability. It also provides (1) a comprehensive list of “trigger items” for
decision support activities and (2) measurable starting points for evaluation.
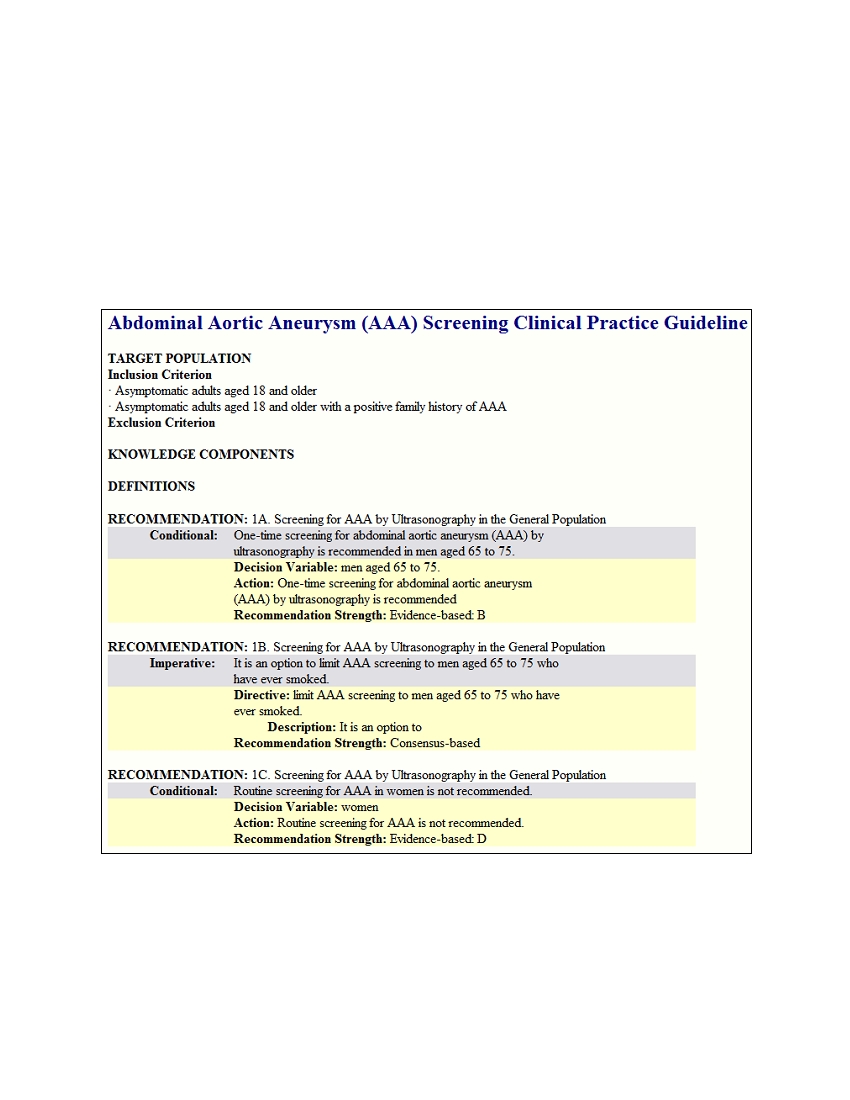
4. Actions . All of the actions (and directives) are removed from guideline context and presented in a list.
This report offers This report offers an enhanced opportunity to judge and identify vagueness,
underspecification, and decidability. It also provides (1) a comprehensive list of activities that will need
to be addressed in the design of decision support systems activities and (2) a listing of potentially
measurable actions.
5. GEM-COGS . This will show the elements in the current GEM II document that satisfy the COGS checklist.
To learn more about COGS, you can visit the web site at http://gem.med.yale.edu/cogs.
6. XML . The last option in the Report menu is “View XML”, which will display the current version of the
XML file. The actual XML report is automatically generated by the GEM-Cutter tool when the project is
saved by the user, and can be found in the folder corresponding to that guideline project (see below).
The format of the output will look similar to that below:

7. Recommendations. This output can be generated by using the Gem Extractor (available at
http://gem.med.yale.edu/GEMTools/gem_ii_tools.htm). In this report the text of the guideline
recommendations is extracted and presented in full text. In effect, this view represents an Executive
Summary of actionable statements from the guideline.
.